Epidemiology and Genomics Research Program
Advancing Cancer Control through the Science of Epidemiology
Foundational epidemiologic research, the scientific bedrock of public health and evidence-based medicine, has contributed to the strides made in reducing cancer incidence and mortality and improving the quality of life after cancer diagnosis and during and after treatment. EGRP, including the Office of the Associate Director and the program’s five branches, supports and advances the science across the cancer control continuum through developing and validating tools to assess exposures, developing research initiatives, managing grants, and facilitating democratization of data access through broad data sharing, collaborative research, and generation and leveraging of existing resources. Members collaborate across the program, division, institute, NIH, and other agencies, in carrying out these activities.
From Etiology to Action
EGRP has been at the forefront of etiologic research, facilitating studies that have identified genetic, constitutional, and environmental factors associated with cancer risk, creating a pathway to prevention, early detection, and improving cancer outcomes. Supported research covers an array of study designs, such as familial and linkage studies, case-control, and prospective cohort studies, that have shed light on understanding the genetic and environmental landscape of risk and how exposures that occur across the lifespan interact to increase the risk for, or protect against, cancer, and provide a road map for cancer prevention and control.
A significant investment in long-term prospective cohort studies for over 40 years has contributed to discovering the determinants of cancer risk. One of the first population-based US cohort studies with a biobank was the CLUE study, established in Washington County, Maryland, in 1974 (Comstock et al), followed in short order by the Nurses’ Health Study, which was initiated in 1976 as a questionnaire-based study with later additions of biospecimen collections. Subsequently, numerous prospective etiologic-focused and survivor-focused cohort studies have been established with NCI funding and serve as valuable resources for cancer-focused research. EGRP has harnessed and leveraged these resources through the NCI Cohort Consortium, described earlier. The consortium provides strength in numbers and fosters pooling projects of particular value in the study of rarer forms of cancer.

Rigor, Reproducibility, and Sharing
The ultimate value of research results depends on the quality of the data captured as well as a rigorous study design. EGRP includes two branches that have a primary focus on developing accurate and reproducible assessment methods and translating those tools to large-scale research endeavors. From single-cell technology, to multiple “omics” assays, to dietary and physical activity assessment measures, advances in technologies and high-quality assessment are supported and made available to researchers. A variety of tools and resources have been developed, maintained, and continue to evolve, with special emphasis in dietary and physical activity assessment.
Data sharing with the democratization of data access enables the totality of research investment to be greater than the sum of its parts. Broad sharing enables students to develop research skills, early-stage investigators to explore research directions and obtain preliminary data, and any investigator to analyze the data in innovative ways and to reproduce findings in similar or different populations. Program members lead efforts across the division and the institute to foster data sharing of NCI-supported research, enabling the leveraging of existing resources to expand knowledge. The program has developed resources to assist the extramural research community in complying with NIH data-sharing policies and enabling broad data sharing.
From Research to Action
Epidemiologic research has identified multiple major causes of cancer incidence, which has led to advances in cancer prevention (such as chemoprevention for breast cancer risk reduction and HPV and hepatitis vaccines to prevent infection-associated cancers), guided additional risk reduction by avoidance of carcinogenic exposures, and provided the evidence for public health and clinical prevention guidelines. While significant progress has been made, more causes remain to be discovered and addressed using the armamentarium of epidemiologic approaches of observational studies and intervention trials.
Looking to the Future
With constant forward motion to accelerate cancer control research, EGRP has launched new initiatives to fund the next generation of cohort studies to investigate environmental risk factors for developing cancer and the cancer survivorship experience, with a particular emphasis on understudied and underserved populations. Simultaneously, EGRP is opportunistic in leveraging emerging, novel, high-throughput, and at-scale approaches including omics to study the cancer continuum in populations. These initiatives, along with leveraging and democratization of existing resources, ensure continued progress in identifying new approaches to cancer control.
Clinical and Translational Epidemiology Branch: Identifying Factors to Improve Outcomes for Cancer Survivors
The Clinical and Translational Epidemiology Branch (CTEB) funds and promotes epidemiologic research to investigate how clinical, genomic, lifestyle, and other factors affect cancer outcomes among cancer survivors, a steadily growing population due to advances in screening and treatment. CTEB also supports the development, evaluation, and implementation of individualized risk prediction models that identify individuals at the greatest risk of treatment-related adverse health outcomes. These efforts advance CTEB’s goal to generate evidence to inform the development of interventional strategies and clinical guidelines and improve the health of cancer survivors.
Epidemiologic research supported by the branch includes both observational and interventional study designs that focus on health effects after a cancer diagnosis. Over the past decade, CTEB has led a major initiative to support the development and growth of more than 10 prospective cohort studies of cancer survivors, investigating multiple factors and their association with short- and long-term health outcomes. Together, these studies include more than 50,000 cancer survivors across more than 12 cancer sites. Most recently, in FY 2021, new cohorts have been funded to fill in evidence gaps related to postdiagnosis cancer outcomes, such as the longer-term health outcomes in cancer patients exposed to newer treatments (e.g., immunotherapy and molecularly targeted therapy); cancer outcome disparities based on race/ethnicity, socioeconomic status, geography, and other factors; and survivors diagnosed with less common cancers.
Long-term side effects of some cancer treatments can impair the length and quality of life of cancer survivors. For example, cancer treatment-related cardiovascular complications, such as heart failure, hypertension, and arrhythmias, remain a leading cause of treatment-associated morbidity and mortality among cancer survivors. Since 2011, CTEB has led a multi-institute initiative across NCI and NHLBI that supports collaborative research on cancer treatment-related cardiotoxicity to address knowledge gaps and identify promising opportunities — including early detection, management, risk prediction, and prevention—stemming from workshops held in 2013 (2013 Workshop) and 2018 (2018 Workshop). CTEB has also led a trans-NCI initiative on the clinical characterization of cancer therapy-induced adverse sequelae and, in 2019, developed a funding announcement to support collaborative research in this area (PAR-19-325).
Another area of focus has been understanding how cancer, cancer treatment, and other factors influence the aging trajectories of cancer survivors. The treatments that spare cancer survivors from mortality may put the cancer survivor at risk for a spectrum of aging-related health conditions at a younger age than would normally occur. In 2018 and 2019, with our partners in BRP, CTEB led two workshops to identify gaps in the area of accelerated aging in cancer survivors. Subsequently, an administrative supplement opportunity was made available, and in partnership with the National Institute on Aging, a Notice of Special Interest (NOSI) was published to encourage research in the following areas: longitudinal studies to examine aging trajectories; studies to elucidate the pathways that lead to aging phenotypes in cancer survivors; and long-term clinical surveillance to monitor survivors for late-emerging effects. Addressing these needs will help inform strategies to optimize healthy aging of cancer survivors.
Lifestyle and Nutrition-related Factors
CTEB supports research to understand how diet, physical activity, and obesity may influence health after cancer treatment. Research results suggest dietary factors and exercise are associated with better outcomes among cancer survivors. A relatively new area of emphasis for CTEB is how body composition (i.e., the proportions of fat and lean mass) affects cancer outcomes, including common adverse events of systemic cancer therapies and survival. Varying levels of muscle and fat may affect the pharmacokinetics of systemic cancer therapies, resulting in life-threatening toxicities from overdosing or poor disease outcomes from underdosing. CTEB supports a webinar series highlighting methodologic challenges and the latest research results, and a range of initiatives that help build the evidence to inform patient management guidelines.
Clinical, Genomic, and Pharmacogenomic Factors
Variation in germline and somatic genomes and clinical and lifestyle factors can influence the development of adverse events, increase risk for certain types of cancers and syndromes, guide treatment decisions, and affect response to therapy. Supported research investigates the association between genomic factors, chemotherapy dosages, lifestyle factors, and severe toxicities, such as neurotoxicity and cardiotoxicity.
Risk Prediction
CTEB also supports the development of individualized risk prediction models that identify individuals at the greatest risk of treatment-related adverse health outcomes. For example, funded studies investigate the genomic and clinical features allowing for the stratification of tumors into those with a good prognosis and those with poor prognosis at the time of diagnosis and thus guiding treatment interventions.
Looking toward the future, CTEB plans to continue to expand its support of studies that identify factors affecting adverse outcomes in cancer survivors, including leveraging existing and completed clinical treatment trials to capture long-term outcomes.
Environmental Epidemiology Branch: Life Exposed —How Our Surroundings Can Impact Cancer Risk
The Environmental Epidemiology Branch (EEB) promotes and supports epidemiologic research on modifiable risk factors and cancer risk in diverse populations to inform and advance the prevention and control of cancer. In accordance with this mission, EEB advances research opportunities to increase understanding of environmental exposures across the life-course on cancer risk in humans. This is illustrated by funding opportunities initiated and led by EEB, such as the Early-life Factors and Cancer Development Later in Life, and by collaborations with other NIH Institutes, such the BCERP and the Global Environmental and Occupational Health (GEOHealth) initiatives.
Initiatives Focused on Understanding of Environmental Exposures and Cancer Risk
The initiative on early-life factors provides key DCCPS-led funding of epidemiologic research to understand how exposures early in life, an understudied area of research, affect cancer risk. BCERP exemplifies a long-standing collaboration with NIEHS to address community-driven efforts to understand whether exposure to environmental factors, in adulthood as well as in early life and developmental periods, are associated with the development of breast cancer. EEB’s collaboration with the Fogarty International Center’s GEOHealth initiative supports research and training for institutions in low- or middle-income countries (LMICs) to tackle environmental and occupational health threats.
To address knowledge gaps in environmental exposures and cancer, EEB has initiated and led a recent funding initiative, New Cohorts for Environmental Exposures and Cancer Risk. This initiative will fund the next generation of prospective cancer cohorts anticipated to have substantial public health impact in assessing environmental exposures, especially environmental chemical and physical exposures, and the risk of cancer, especially in underserved and understudied populations. These new cancer epidemiology cohorts are expected to become national resources, leveraged by the research community to reduce risk, incidence, and deaths from cancer as well as enhance the quality of life for cancer survivors, the division’s overarching mission.
EEB is actively collaborating with the NIEHS-led Human Health Exposure Analysis Resource initiative that enables cost-effective laboratory measurements of novel environmental exposures. This is another example of EEB’s efforts to fund grants that apply novel approaches and techniques to advance research on the exposome and cancer risk either through directly leading or joining with other NIH initiatives. The staff of EEB also actively collaborate with NIH’s Environmental Influences on Child Health Outcomes (ECHO) program, which addresses research at the intersection of pediatric and environmental health.
Broadening Research in Diverse, Understudied, and Underrepresented Populations
EEB continues efforts to broaden research in diverse human populations. Members of the branch are involved in a plethora of funding opportunities focusing on risk factors or cancers that disproportionately affect understudied and underrepresented populations, including those living in certain geographic locations or the important factors contributing to the increase in AIDS-defining and non-AIDS defining cancers. The branch continues to apply novel approaches and techniques to advance research on the environment and cancer risk, for example, in areas related to geospatial methods, exposomics, metabolomics, and microbiomics. EEB also actively engages community partners to identify relevant scientific focus and recruitment of hard-to-reach populations to scientific studies. The areas highlighted in this overview represent a research snapshot of activities that EEB is involved with as a branch to realize its vision of eliminating environmentally induced cancers for the well-being of all populations.
Looking Forward
As EEB looks to the near future, the branch will advance current efforts to expand its focus to other novel exposures/factors (e.g., cannabis, novel pathogens, e-cigarettes, climate change, chemical toxicants, co-morbid conditions, social constructs), to evaluate the notably complex interactions among exposures and the subsequent risk of cancer, and to broaden research across diverse populations. These ongoing efforts capitalize on the branch’s scientific and programmatic expertise and knowledge base to timely and collaboratively address contemporaneous issues. For example, the legal landscape of medical and recreational cannabis use is rapidly evolving. Several efforts across NCI are underway to understand the scientific landscape regarding the risks and benefits of cannabis use. EEB led the 2020 symposium on cannabis and cannabinoids and cancer, which highlighted the state of the science and research gaps. Likewise, as the obesity epidemic continues and the evidence of its etiologic association with multiple cancers is solidifying, EEB is leading a cross-NCI initiative to establish a transdisciplinary consortium of funded research projects to understand the underlying mechanisms of obesity-associated cancer risk. EEB members are also engaged in collaborations with partners across NIH to address the challenge of climate change and cancer. These examples serve to highlight a multitude of planned and ongoing activities that will chart EEB’s scientific engagement in the near-term and beyond.
Genomic Epidemiology Branch: Understanding the Inherent Risk Factors of Cancer
The overarching goal of the Genomic Epidemiology Branch (GEB) is to elucidate the genetic architecture of cancer, improve our understanding of the genetics of cancer, and enhance its potential to enable effective disease prevention, treatment, and survivorship. Since the completion of the Human Genome Project in 2003, technological advances have revolutionized our ability to catalogue both common and rare genomic variation. This has led to a greater appreciation of cancer as a complex disease caused by many genetic and environmental factors working together, with few, if any, being absolutely required for disease to occur. This means that jointly modeling genetic, environmental, and lifestyle-related exposures promoting cancer is critical to understanding the role of genetics in cancer risk, and cancer health drivers.
GEB’s scientific priorities include novel genetic discovery, understanding the role of inherited genetic variants in cancer biology, and enabling advances that benefit all. Further, leveraging existing resources, ensuring rigor and reproducibility, and promoting innovative study designs and analytic approaches drive the branch’s activities.
Driving Discovery of New Genetic Risk Loci by Leveraging Existing Resources, Sharing Data, and Collaborating Across Disciplines
Each of these priorities is evident in the foundational branch-led Genetic Associations and Mechanisms in Oncology (GAME-ON) initiative (RFA-CA-09-002). Specifically, the GAME-ON initiative, including the OncoArray consortium, built on NCI’s previous investment in cancer epidemiology studies as well as existing genome-wide genotyping data, have given rise to some of the largest collections of cancer genomic risk data. Notably, pooling data on this large scale (33 studies; 500,000 samples; and 128 investigators) empowered both the replication of previous findings as well as the discovery of new risk loci. The GAME-ON initiative led to many new partnerships and investigators from more than 350 institutions, 60 countries, and multiple research areas collaborating to discover hundreds of novel variants associated with cancer predisposition. To date, the GAME-ON initiative has led to more than 400 publications.
All of the data generated by the GAME-ON initiative is currently available for secondary analyses via NIH’s database of Genotypes and Phenotypes (dbGaP). Notably, many investigators have leveraged these data to formulate new hypotheses that are being investigated using additional NIH funding. This work has often been supported by branch-developed funding opportunity announcements (PA-17-239, PA-17-243, PAR-20-277, PAR-20-276) focused on secondary analysis and integration of existing data to elucidate the genetic architecture of cancer risk and related outcomes.
Investments in Functional Genomics Methodologies
A critical step in understanding the genetic architecture of cancer is narrowing an implicated locus to a set of genetic variants that directly cause an increased cancer risk by disrupting the expression or function of a protein. Functional genomics highlights the need to foster collaboration between basic cancer biologists and genetic epidemiologists, as well as the integration of many data types, including genomics, metabolomics, transcriptomics, and epigenomics, to gain insight into disease mechanisms. GEB has invested heavily in metabolomics as a functional genomics methodology. The primary metabolomics initiative managed by the branch is the COnsortium of METabolomics Studies (COMETS). Many COMETS activities, such as COMETS Analytics, focus on ensuring rigor and reproducibility in the application of this new technology to cancer epidemiologic studies.
Improving Inclusion of Diverse Populations in Research Studies
While progress has been made towards improving the inclusion of diverse populations, racial and ethnic minorities remain underrepresented in cancer genomic epidemiologic studies. This inadequate representation limits the translational impact of findings on these populations. The GAME-ON initiative included ~200,000 samples from previously understudied populations and helped identify cancer risk variants across and unique to different populations. GEB continues to build rich genomic resources and promote the inclusion of diverse populations in genetic epidemiologic research. Current efforts include “Genome-wide Genotyping of Existing Samples from Minority Racial/Ethnic Populations and Sharing of Associated Epidemiologic Data” (NOT-CA-21-049), which will leverage existing biospecimens to diversify germline genome-wide genotyping data repositories and further enhance them with accompanying exposure, phenotype, and outcome data. These data resources will allow the study of genetic cancer susceptibility in diverse populations, to better understand its role in cancer health drivers.
Integrating Innovative Study Designs and Analytical Approaches
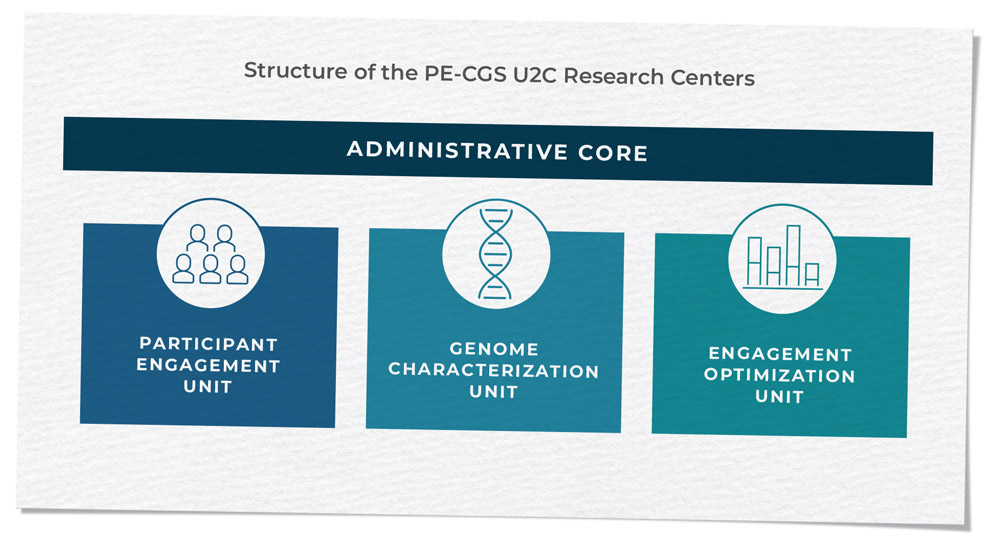
Innovative study designs and analytic approaches are essential to drive genomic discoveries and examine inherited genetic variation within a broader context considering gene-gene or gene-environment interactions, as well as interactions between the germline and somatic genomes (as explored through PQ3 in RFA-CA-17-017). Many branch activities are driven by innovation, and this is exemplified most recently by the Cancer Moonshot-supported Participant Engagement and Cancer Genome Sequencing (PE-CGS) Network (RFA-CA-19-045 and RFA-CA-19-046). The PE-CGS Network employs innovative ways to directly engage cancer patients and post-treatment cancer survivors as participants in rigorous cancer genome sequencing programs, addressing important knowledge gaps in the genomic characterizations of understudied tumors.
Through these initiatives and the support of our extramural researchers, GEB is shaping the research landscape on the genetic, epigenetic, immunological, and biological factors that influence cancer risk and maximizing its scientific potential.
Methods and Technology Branch: Fostering Rigor and Reproducibility
The transition of assays from small-scale applications to large-scale population studies requires high-throughput procedures that maintain high standards of reproducibility and validity and low cost. The Methods and Technologies Branch (MTB) supports the adaptation of laboratory and technical approaches to large-scale human population studies, ensuring the validity of tests used in population studies. During the last decade, efforts were successfully made in incorporating epigenomics, metabolomics, transcriptomics, proteomics, and microbiome in large-scale population-based studies relevant to multiple stages across the cancer control continuum.
Applying Omics Approaches to Large-Scale Studies
A prototypic example of MTB’s role in facilitating technological applications in large-scale studies is the use of epigenomics in research studies. Epigenetic changes are essential for normal development, and their abnormal expression may result in the initiation or progression of different cancer types. Both DNA sequence-based genetic changes (mutations, polymorphism, deletions, additions, translocations) and epigenetic changes may alter gene expression. Through MTB-initiated and collaborative funding announcements across NCI and NIH, the branch has supported research to help determine the role of epigenetic changes in developing cancer risk and identification of genetic, environmental, and host susceptibility factors that modify the risk in different populations by capitalizing on approaches for profiling of methylation patterns, histone modifications, and microRNAs. Further, through participation in the Common Fund Epigenomics program, the branch is leveraging opportunities to identify cancer-associated epigenetic marks. In association with the National Institute on Minority Health and Disparities, MTB participated in initiatives on social epigenomics focused on minority health and health drivers. These initiatives are especially significant because individuals living in disadvantaged neighborhoods are often exposed to social (food desert, violence or threat of violence, discrimination, residential segregation, and psychosocial stress), chemical, and physical (noise pollution, lack of green space) stressors. Ultimately, this line of research may contribute to new approaches to reduce health drivers.
MTB staff represent NCI in many trans-NIH initiatives, such as the congressionally mandated ECHO program focused on supporting existing cohorts to address how pre, peri, and postnatal environmental exposures impact childhood development and health outcomes. This initiative leverages existing populations and emerging technological resources to study environmental factors and associations with outcomes that have a high public health impact. In addition to ECHO, MTB staff represent NCI in many NIH Common Fund initiatives in epigenomics, metabolomics, and molecular transducers of physical activities and contribute to developing molecular approaches in these emerging areas of research.
Developing and Improving Technologies for Cancer Control and Population-Based Research
In parallel to the trans-NIH initiatives, MTB participates in multiple collaborative trans-NCI initiatives focused on technological advances to study mechanisms of carcinogenesis, as well as validation of assay technologies for high-quality markers in clinical studies to assess their utility in cancer detection, diagnosis, and treatment. Through collaboration with the Center for Global Health, MTB has promoted initiatives to develop cost-effective technologies that can be implemented in low-resource settings. Further, MTB staff have collaborated for many years with NCI’s Small Business Innovation Research (SBIR) program, developing topics for SBIR solicitations aimed at supporting research and development of new or improved technologies and methodologies that pertain to cancer control and population sciences and have the potential to succeed as commercial products. Examples of SBIR topics include high-throughput technologies for simultaneous isolation and analysis of exosomes, the development of single-cell “unbiased discovery” proteomic technologies, multiplex technologies for screening and monitoring cancer-associated co-infections at the point of sample collection, next-generation 3D tissue culture systems with tertiary lymphoid organoids, and direct sequencing of nucleic acids without clonal amplification or synthesis.
Through its initiatives and extensive collaborative activities across NCI and NIH, MTB serves the research community at large to ensure the validity and reproducibility of emerging technologies in large-scale studies. Notable recent achievements that accelerated research activities included techniques to leverage the value of archival tissue, multi-omic technologies (genomics, epigenomics, metabolomics, microbiome, transcriptomics, and proteomics), and the development of software and tools for next-generation heat maps for fluent, interactive exploration of data. Looking toward the future, MTB sees the expansion of emerging techniques, such as single-cell technologies, and will continue to support the necessary research to ensure their validity as they expand to application in large-scale population-based studies.
Risk Factor Assessment Branch: Innovating Assessment of Diet, Physical Activity, and Sleep Across the Cancer Continuum
The Risk Factor Assessment Branch’s (RFAB) innovation in diet and physical activity assessment provides a critical link between research and translation into guidance and interventions for cancer prevention and control. RFAB develops, supports, and stimulates assessment of modifiable risk factors among individuals and diverse populations across the cancer continuum to inform and advance health promotion.
Providing Tools and Developing Metrics
RFAB has revolutionized the field of diet assessment and helped change the way we think about diet, pioneering a holistic view that considers and analyzes dietary patterns, not only individual nutrients, and incorporates multilayered contextual factors that vary throughout a person’s life. RFAB and collaborators developed a freely available tool, the Automated Self-Administered 24-Hour (ASA24®) Dietary Assessment Tool, which enables research participants to report what they have consumed within the last 24 hours or in real time as a food record (Subar et al). ASA24 provides information that previous tools do not, such as when food was eaten, where, with whom the food was eaten, and with what other foods and beverages. RFAB has advanced the discourse on dietary patterns and partnered with USDA to develop and evaluate the Healthy Eating Index (HEI) (Krebs-Smith et al), a measure of alignment with the Dietary Guidelines for Americans (DGAs). By supporting researchers to apply the HEI as a measure of the totality of the diet and standardizing analyses in large cohorts through the Dietary Patterns Methods Project, research on dietary patterns has been transformed.
Supporting Data and Infrastructure
RFAB has facilitated advances in the assessment of physical activity research by promoting and supporting the inclusion of physical activity assessment with devices in the National Health and Nutrition Examination Survey (NHANES). Publicly available data from the waist-worn accelerometers deployed in NHANES 2003–2006 have been the basis of more than 80 research publications, and the initial 2008 paper describing these data (Troiano et al) has been noted as one of five most influential papers in physical activity research (Varela et al). Building on technology advances, RFAB led an effort to include wrist-worn accelerometer devices in NHANES 2011–2014 and the NHANES National Youth Fitness Survey. These data, based upon 24-hour wear, will provide a rich resource to study physical activity as well as sleep among children, adolescents, and adults. RFAB staff have also developed population reference curves for the daily accelerometer metrics for use by researchers. Ongoing efforts focus on harmonizing behavioral labelling to support data pooling and to develop algorithms to classify movement behaviors from wrist-worn accelerometer device signal data.
Promoting Synergistic Thinking
RFAB has nurtured partnerships with multidisciplinary teams to advance new analytical methods and directions in assessment. This includes novel insights into the types and extent of measurement error in self-reported diet and physical activity data, and efforts to reduce them, and usual intake modeling of foods across the lifespan to inform guidance. Additionally, RFAB has partnered in collaborations resulting in NCI funding for obesity policy research and development of time-sensitive initiatives to evaluate natural experiments (PAR-18-854, NOT-DK-20-035), and, with NCCOR, to prioritize next steps for measurement needs to spur progress in reducing childhood obesity by creating the Measures Registry Resource Suite and Catalogue of Surveillance Systems.
RFAB’s leadership has been essential to NCI’s contributions to the 2020-2030 Strategic Plan for NIH Nutrition Research, the first NIH-wide strategic plan for nutrition research that presents a vision for the next decade of NIH-supported nutrition research. Aligning with this plan, RFAB will support the Dietary Assessment Center (RFA-RM-21-004) to examine ASA24 and other innovative approaches in the Nutrition for Precision Health powered by All of Us Research Program and accelerate improvements in diet assessment.
International partnerships allowed RFAB staff to support the development of the WHO 2020 Guidelines on Physical Activity and Sedentary Behaviour (2020) and lead the development and standardization of a new score based on cancer prevention recommendations with the World Cancer Research Fund International/American Institute for Cancer Research. RFAB staff served as Executive Secretary for the 2008 and 2018 Physical Activity Guidelines for Americans and on the Policy Writing Group for the 2015 and 2020 DGAs.
Future efforts are focused on innovative strategies to integrate assessment of 24-hour behavior patterns, to help answer questions that single approaches cannot answer. Supporting research that combines self-report diet, physical activity, and/or sleep questionnaires with device-based images, movement sensors, geospatial, and time-stamped data can create a more complete picture for new developments in predictive models with enhanced validity and opportunities for guidance and interventions for cancer prevention. To continue to move these efforts forward, RFAB will further enhance its collaborations within NCI and with other NIH institutes and centers, government agencies, and public-private partnerships. Going forward, further systems-focused efforts will integrate multidimensionality and dynamism not only within these risk factors, but across risk factors, including sedentary behavior and sleep, and yield insights into differences among population subgroups, environmental and other external influences, and interventions designed to change or improve these behaviors.
Footnotes
Comstock GW, et al. The Washington County Training Center: an exemplar of public health research in the field. Am J Epidemiol. 1991 Nov 15;134(10):1023–9.
Subar AF, et al. Performance and feasibility of recalls completed using the Automated Self-Administered 24-Hour Dietary Assessment tool in relation to other self-report tools and biomarkers in the Interactive Diet and Activity Tracking in AARP (IDATA) Study. J Acad Nutr Diet. 2020 Nov;120(11):1805–1820.
Krebs-Smith SM, et al. Update of the Healthy Eating Index-2015. J Acad Nutr Diet. 2018 Sep;118(9):1591–1602.
Troiano RP, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008 Jan; 40(1):181–188.
Varela AR, et al. Mapping the historical development of physical activity and health research: a structured literature review and citation network analysis. Prev Med. 2018 Jun;111:466–472.
Continue To

